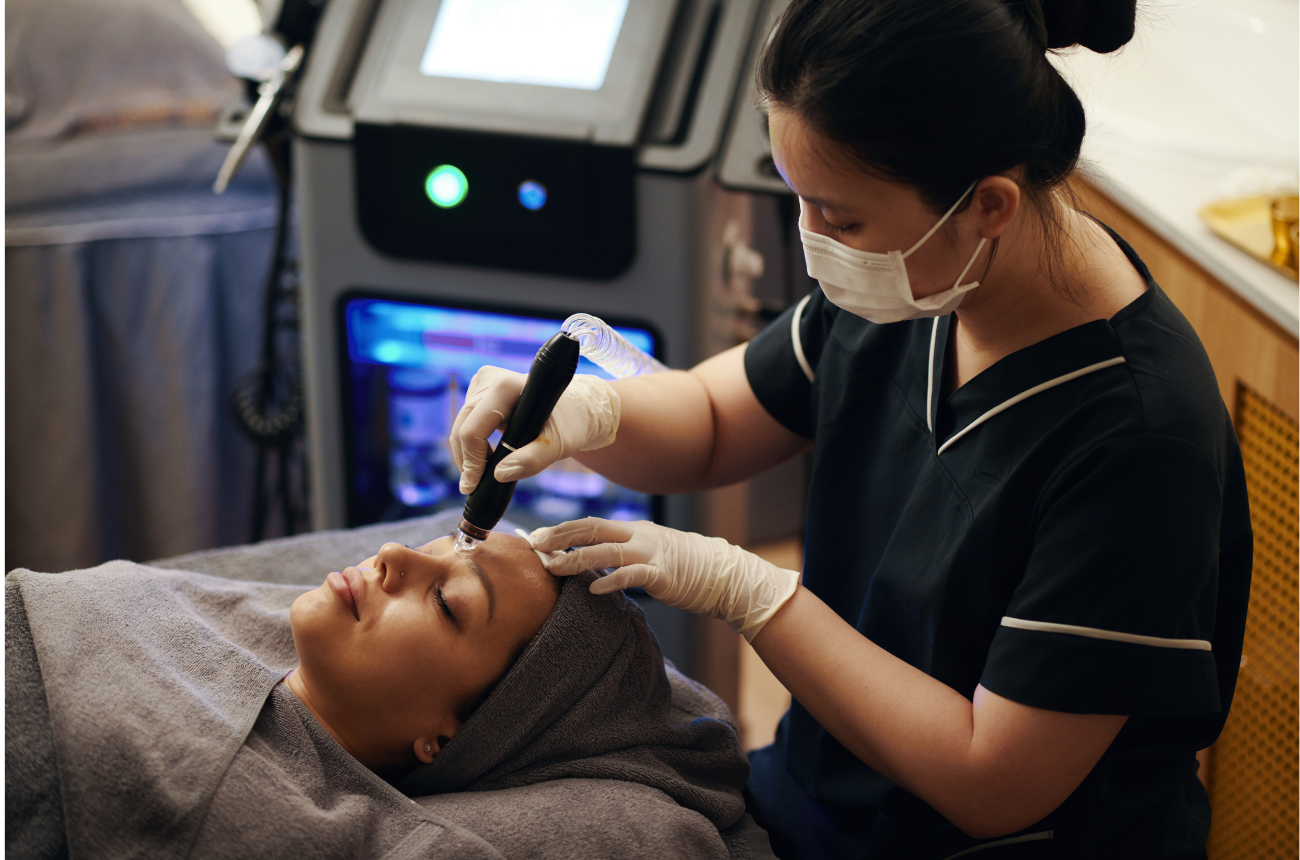
The U.S. Food and Drug Administration (FDA) recently issued a safety communication regarding radiofrequency (RF) microneedling devices, warning of potential risks when these treatments are performed without appropriate medical oversight or training. For medical spa professionals, this is an important reminder that energy-based skin treatments—while popular and profitable—are medical procedures that demand clinical expertise, clear protocols, and ongoing vigilance.
Understanding RF Microneedling
RF microneedling, sometimes referred to as “collagen induction therapy,” combines two techniques: traditional microneedling to create controlled micro-injuries in the skin and radiofrequency energy to heat deeper layers of tissue. This combination is designed to enhance collagen remodeling, improving skin tone and texture over time.
However, the FDA has reported serious complications including burns, scarring, and nerve damage. This places greater responsibility on medical spa professionals to ensure treatments are performed safely, compliantly, and under appropriate medical direction.
Why Clinical Oversight Matters
RF microneedling is a medical treatment, not a cosmetic service. Due to this, licensed healthcare practitioners are required to determine whether a patient is an appropriate candidate for RF microneedling and oversee all aspects of care.
Every reputable medical spa should have:
- A medical director who provides clinical oversight and establishes treatment protocols
- Good faith exams to confirm patient suitability before any procedure
- Proper delegation and supervision processes, based on state laws
- Thorough documentation of training, consent, and post-treatment care
These safeguards protect both patients and practitioners, ensuring treatments are delivered safely and ethically.
Who Can Perform RF Microneedling?
Scope-of-practice regulations vary by state, but in most cases, RF microneedling may only be performed by or under the direct supervision of a licensed medical professional. This typically includes physicians, nurse practitioners (NPs), or physician assistants (PAs). Delegation to nurses or aestheticians may be permitted in some states—but only if proper oversight and protocols are in place.
Before introducing RF microneedling or any new energy-based device to your practice, always:
- Review state medical spa regulations
- Verify device clearance and manufacturer guidelines
- Document training and competency for each team member performing or assisting with the procedure
Lessons for the Aesthetic Industry
The FDA’s alert highlights a growing challenge in aesthetic medicine: device innovation often outpaces clinical regulation and provider education. As new technologies enter the market, ensuring they are used responsibly and within scope is essential for maintaining patient safety and professional credibility.
For practice owners, this means:
- Prioritizing staff training and certification before offering new treatments
- Using only FDA-cleared medical devices
- Maintaining clear treatment protocols and documentation
Supporting Safe, Compliant Aesthetic Care
The FDA’s alert on RF microneedling is a reminder that technology and patient care must move hand in hand. OptiMantra helps medical spa professionals maintain detailed treatment documentation, streamline consent and follow-up workflows, and uphold clear medical oversight for every procedure.
Empower your practice with confidence. Sign-up for a free trial or book a demo to learn more about OptiMantra’s medical spa solutions.
Disclosure: This article is for informational purposes only and does not constitute medical or legal advice. Providers should review FDA communications and state regulations before performing or delegating RF microneedling procedures.